For some using herbal, DEET-free bug repellent is a preference, maybe simply for the sake of using essential oils instead of wearing a DEET cologne. For others there is likely a concern that DEET is at least mildly toxic. Sometimes DEET is used lackadaisically. The purpose of the post is to give a reminder that DEET can be dangerous if used improperly. As a warning there are some examples of animal testing in this post below in the green box.
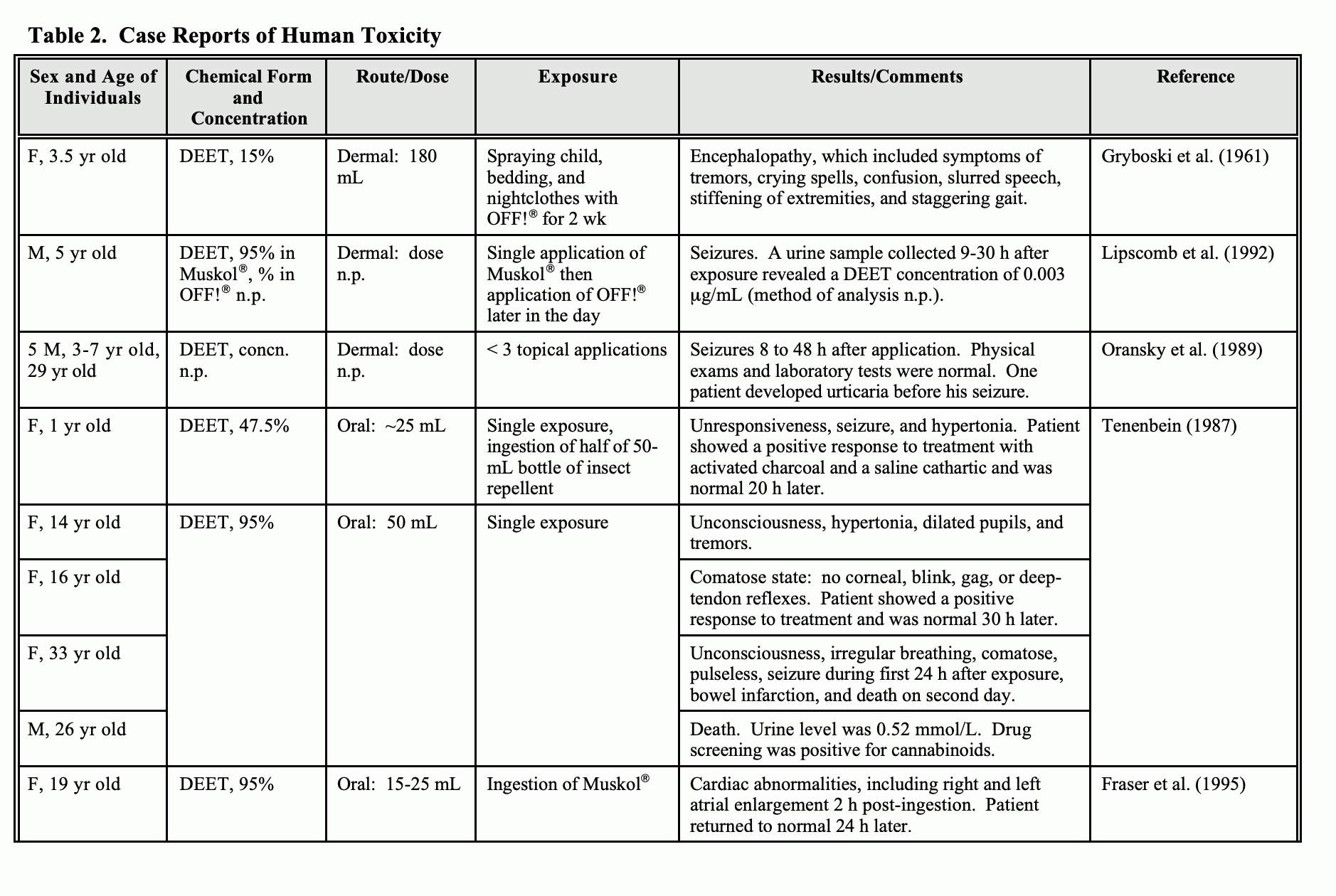
Concern may be stirred by the caution statements on DEET products “If swallowed: Call a poison control center or doctor immediately for treatment advice. … If you suspect a reaction to this product, discontinue use, wash treated skin, and call your local poison control center.” Ingestion of 47.5% to 95% DEET products has led to seizures and coma within 30-60 minutes of ingestion, hypotension and death from the rapid onset of reactions. (1) In addition to the above, the signs and symptoms of DEET toxicity can include bradycardia, confusion, urticaria, abdominal pain, nausea, vomiting, skin irritation, contact rash, acute psychosis, and a burning sensation of the eyes, lips, tongue, and mouth (4).
Research indicates that DEET is relatively safe when used as the instructions recommend. (2) Support of DEET use is largely in the prevention of West Nile virus infection and Malaria. In a study that analyzed 20,764 DEET exposures nearly 70% of the cases reported no symptoms, meaning just above 30% did report symptoms, and 26 reported major effects. Infants and children accounted for the highest number of exposures but had lower rates of adverse reactions than adults and teens. (4)(5) Koren, Matsui and Bailey concluded that evaluation of existing evidence does not support the idea that neurotoxic effects of DEET affect children more than adults and report that a 10% DEET product provides up to three hours of protection, which is more than any non-DEET repellants available. (2) This may provide some peace of mind when in dire buggy circumstances.
The EPA has released the following statements on DEET:
The Agency has not identified any risks of concern to human health, non-target species or the environment. We continue to believe that the normal use of DEET does not present a health concern to the general population, including children.
The human health risk assessment concluded that there are no risks of concern because no toxic effects have been identified when used as a dermally applied insect repellent, and there is no dietary or occupational exposure for DEET.
DEET is approved for use on children with no age restriction. There is no restriction on the percentage of DEET in the product for use on children, since data do not show any difference in effects between young animals and adult animals in tests done for product registration. There also are no data showing incidents that would lead EPA to believe there is a need to restrict the use of DEET.
EPA requires all DEET product labels include the following directions to help reduce the chance of DEET irritating your skin or eyes:
- Read and follow all directions and precautions on this product label.
- Do not apply over cuts, wounds, or irritated skin.
- Do not apply to hands or near eyes and mouth of young children.
- Do not allow young children to apply this product.
- Use just enough repellent to cover exposed skin and/or clothing.
- Do not use under clothing.
- Avoid over-application of this product.
- After returning indoors, wash treated skin with soap and water.
- Wash treated clothing before wearing it again.
- Use of this product may cause skin reactions in rare cases.
The following additional statements will appear on the labels of all aerosol and pump spray formulation labels:
- Do not spray in enclosed areas.
- To apply to face, spray on hands first and then rub on face. Do not spray directly onto face.
The Center for Disease Control and Prevention (CDC) reports more conservatively: “Using insect repellents containing DEET should not be harmful if label directions are followed and the product is used safely.” The CDC gives the following guidelines for safety:
- Read and follow all directions and precautions on the product label.
- Store DEET out of reach of children.
- To apply to face, first spray product onto hands, then rub onto face.
- Use only when outdoors and wash skin with soap and water after coming indoors.
- Higher concentrations of DEET may have a longer repellent effect, however, concentrations over 50% provide no added protection.
- Use just enough repellent to cover exposed skin and/or clothing.
- Avoid over-application of the product.
- DEET may be used on adults, children, and infants older than 2 months of age. Protect infants from mosquito bites by using a carrier draped with mosquito netting with an elastic edge for a tight fit.
Be safe with DEET :
- Do not allow children under 10 years of age to apply repellent themselves.
- Do not apply to young children’s hands or around eyes and mouth.
- Do not breathe in, swallow, or get into the eyes (DEET is toxic if swallowed.)
- Do not put repellent on wounds or broken skin.
A Review of Toxicological Literature on DEET was published in 1999. Here are some animal testing examples that appear to have negative results from studies included in this review with commentary on doses and human comparisons. Note: A lot of the studies are done with high doses of DEET.
“In short-term and subchronic studies, rats given daily oral doses of 750 mg/kg (3.92 mmol/kg) for 21 days exhibited symptoms including hypoactivity, ataxia, decreased muscle tone, foot splay, urine stains, perinasal encrustation, and perioral wetness.” Rats weigh about 300g or .3kg on average, in which case a dose of 750 mg/kg is 225mg. Following 750mg/kg, a person weighing 60 kg (132lb) would be eating about 45 grams or 1.6 oz a day.
“Among female Sprague-Dawley rats exposed subcutaneously (s.c.) to 0.3-1.80 mL/kg (1.6-9.4 mmol/kg), none survived more than 10 days and liver and kidney weights were elevated. Male rats in the same study developed skin lesions at the injection site and showed gait disturbance; autopsy revealed grossly distended, urine-filled bladders.”
“Rabbits experienced edema of the nictitating membrane, lacrimation, conjunctivitis, purulent discharge, and occasional corneal cloudiness when exposed ocularly to 10-100 µL of 100% DEET.”
“Rabbits exposed orally to 132-528 mg/kg (0.690-2.76 mmol/kg) DEET showed decreased body weight and increased kidney weight; serum calcium levels decreased while cholesterol and triglyceride levels increased.”
“The skin of rabbits exposed dermally for 14 days had increased foldings and indentations on the skin surface, thinning of the epidermis, and cystic dilations of the dermis.”
"Dogs and cats given either oral or dermal doses of 0.089-7.128 g/kg (0.46-37.26 µmol/kg) of a flea and tick spray containing 9% DEET experienced ataxia, seizures, muscle tremors, hypersalivation, restlessness, depression, and incoordination."
"No significant reproductive or developmental effects were observed in the fetuses of female and male rats or rabbits given DEET. In chick embryos, however, gross malformations, including ventricular septal defects, anomalous aortic arch patterns, absence of a rump, absence of or malrotated limbs, and central nervous system (CNS) malformations were seen in those exposed to 1.27 µmol (243 µg) DEET by topical application to the chorioallantoic membrane (around fetus) on the second day of incubation.”
The following excerpts regarding human pregnancy are in the Review mentioned above: “The first study of the safety of DEET when used regularly during the second and third trimesters was a randomized, double-blind trial involving 897 pregnant women in Thailand who continuously applied therapeutic doses of DEET topically (1.7 g/d) — a dose similar to that recommended to prevent malaria — or placebo to prevent malaria. … In the group as a whole, no adverse neurological, gastrointestinal or dermatological effects were observed in the women exposed to DEET, and no adverse effects on survival or growth and development at birth and at 1 year of age were detected in the babies whose mothers used DEET.”
“We found no human studies of exposure to DEET in the first trimester. However, the very high dose administered orally in the animal study suggests that DEET is safe when used as recommended.”

For humans, “Applying 10–12 g of a 75% DEET solution to the skin can lead to a blood concentration of about 0.0005 mmol/L; ingestion of a similar amount of DEET can result in a blood concentration that is hundreds of times higher (1 mmol/L).” (2) In humans, a blood concentration of 1 mmol/L has been associated with seizures and death.(2) In one case death resulted from ingestion of 6 oz of 40% DEET. (6) So, applying topically results in much lower blood concentrations than eating it – which can easily be concluded.
The lessons: really avoid ingesting any DEET! One should probably also avoid inhaling it. In small, acceptable topical amounts there aren’t many adverse reactions reported. Overall, it seems wise to use it only as needed, treat it with respect and use it as the CDC has suggested above: apply it carefully, use on children older than 2 months, and consider using products of less than 50% DEET.
If you’d like to do more of your own research, here are two Toxicological reports.
Review of Toxicological Literature
Toxicological Profile for DEET published by the U.S. Dept of Health and Human Services, Agency for Toxic Substances and Disease Registry August 2017. This is incredibly thorough.
- Tenenbein, M. “Severe Toxic Reactions and Death Following the Ingestion of Diethyltoluamide-Containing Insect Repellents.” JAMA: The Journal of the American Medical Association, vol. 258, no. 11, 1987, pp. 1509–1511., doi:10.1001/jama.258.11.1509. https://www.ncbi.nlm.nih.gov/pubmed/3625951
- Koren G, Matsui D, Bailey B. DEET-based insect repellants: safety implications for children and pregnant and lactating women. CMAJ 2003;169(3):209-12. [PMC free article] [PubMed]
- Bell, John W., et al. “Human Exposures to N, N-Diethyl-m-Toluamide Insect Repellents Reported to the American Association of Poison Control Centers 1993–1997.” International Journal of Toxicology, vol. 21, no. 5, 2002, pp. 341–352., doi:10.1080/10915810290096559. [PubMed]
- Leikin, J. B., and F. P. Paloucek (Eds.). 1998. Poisoning and Toxicology Compendium. Cleveland, OH: Lexi-Comp, Inc., pp. 677-678.
- Tice, R., Brevard, B. March 1999. N,N-Diethyl-m-toluamid (DEET) Review of Toxicological Literature.
These statements have not been evaluated by the Food and Drug Administration.
This information and these products are not intended to diagnose, treat, cure or prevent any disease.